Chronic lung disorders: study of the biochemical features of proteins involved during inflammation and development of new therapeutic interventions
Chronic lung disorders: study of the biochemical features of proteins involved during inflammation and development of new therapeutic interventions
Research Group: Simona Viglio, Paolo Iadarola, Maura D’Amato
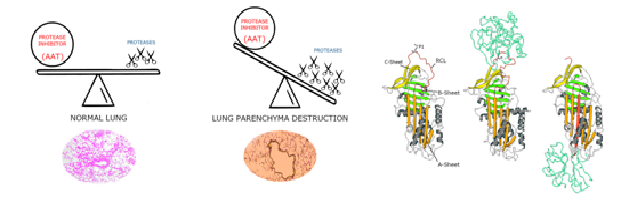
Neutrophils play a critical role in the immune response to chronic lung disorders (CLD), contributing to lung tissue damage, inflammation, and disease progression. The massive neutrophil activation results in the release of various harmful factors, including proteases (mainly human neutrophil elastase, HNE), myeloperoxidase, and free radicals, having a detrimental effect on lung extracellular matrix. Alpha-1 antitrypsin (AAT), the natural HNE inhibitor, is crucial in protecting the lung extracellular matrix from damage. In fact, under normal conditions HNE is balanced by an equivalent level of AAT, through the formation of a stable covalent complex. However, during inflammatory and infectious processes, HNE excess is produced triggering persistent inflammation and lung tissue damage. Furthermore, AAT may fail in its inhibitory role because of oxidation caused by the severe hypoxic environment in the lung. Therefore, developing therapies that preserve or restore functional AAT activity could offer new strategies for managing CLD and improving patient outcomes. Given HNE’s role in various severe lung disorders, its regulation presents a promising therapeutic target.
In this context our research group is mainly focused on:
1. Investigating the AAT oxidation state in biological fluids of patients affected by CLD .
2. Evaluating the effects of two non-peptide HNE inhibitors, Sivelestat and Alvelestat, on both standard and endogenous HNE, by using a combination of in silico computational modeling and in vitro enzymatic assays aimed at monitoring HNE activity.
3. Designing and synthesizing new HNE-specific inhibitors that could serve as novel targeted therapies against lung damage.
4. Producing recombinant AAT-like proteins resistant to oxidative stress while simultaneously retaining their biological role.
Recent Publications:
- D’Amato M, Campagnoli M, Iadarola P, Bignami PM, Fumagalli M, Chiarelli LR, Stelitano G, Meloni F, Linciano P, Collina S, Pietrocola G, Vertui V, Aliberti A, Fossali T, Viglio S. Could the Oxidation of α1-Antitrypsin Prevent the Binding of Human Neutrophil Elastase in COVID-19 Patients? Int J Mol Sci. 2023;24(17):13533.
- D’Amato M, Vertui V, Pandolfi L, Bozzini S, Fossali T, Colombo R, Aliberti A, Fumagalli M, Iadarola P, Didò C, Viglio S*, Meloni F*. Investigating the Link between Alpha-1 Antitrypsin and Human Neutrophil Elastase in Bronchoalveolar Lavage Fluid of COVID-19 Patients. Curr Issues Mol Biol. 2022;44(5):2122-2138.
- Cagnone M, Piloni D, Ferrarotti I, Di Venere M, Viglio S, Magni S, Bardoni A, Salvini R, Fumagalli M, Iadarola P, Martinello S, Meloni F. A Pilot Study to Investigate the Balance between Proteases and α1-Antitrypsin in Bronchoalveolar Lavage Fluid of Lung Transplant Recipients. High Throughput. 2019;8(1):5.
